Muscle and fat are the body’s only organs that are dynamically regulated in size—growth and reduction in size are affected by the combination of nutrient intake, exercise, growth factors, and activin signaling.
Versanis is the first to tackle obesity by targeting activin type II receptors (ActRII), the activin receptors found in both fat and muscle cells. Signaling through ActRII receptors causes wasting in muscles and storage of fat in adipose tissue. Human genetic studies have implicated members of the activin pathway in obesity.
Bimagrumab’s Unique MoA
Mechanism of action in obesity
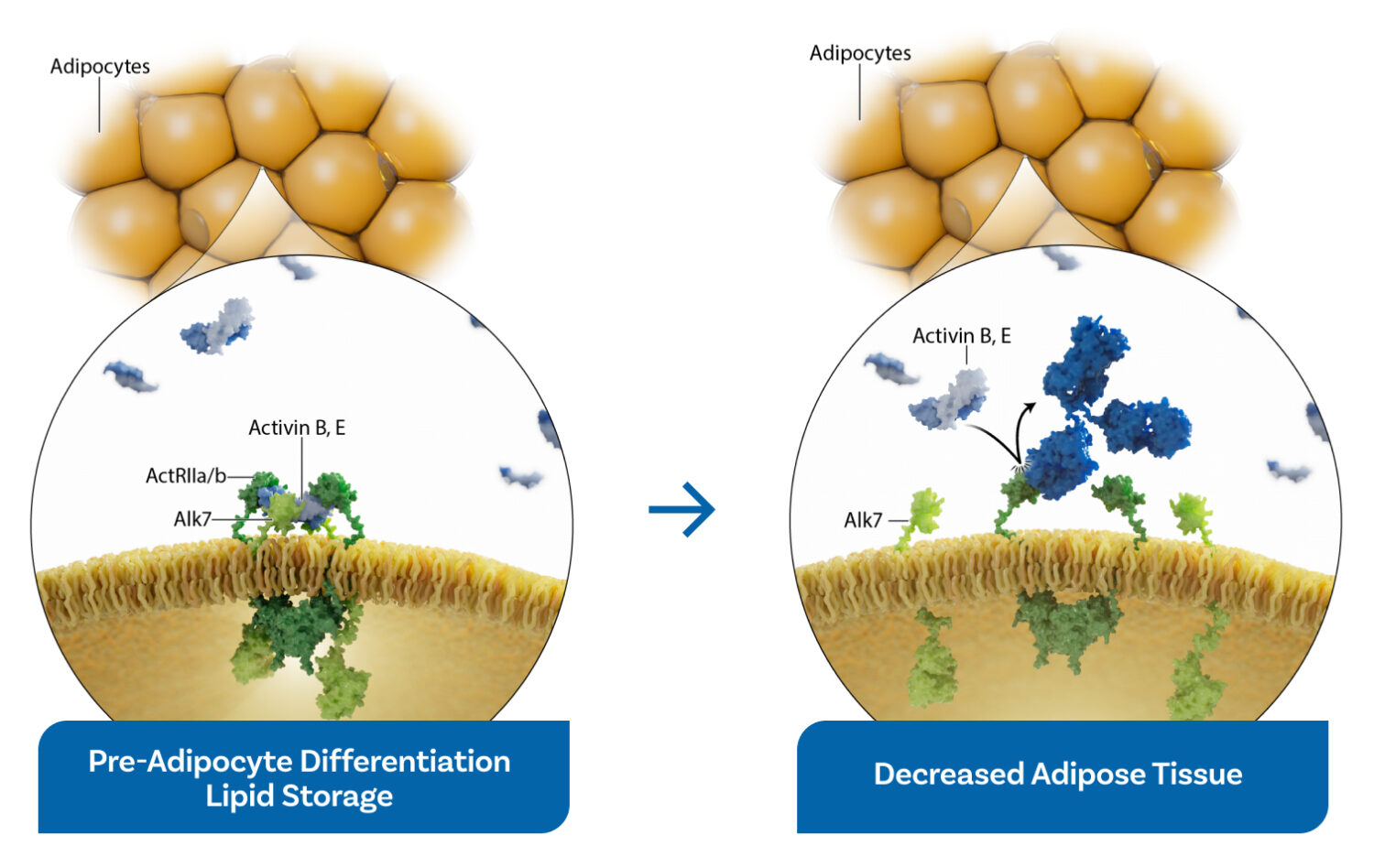
ActRII biology in adipose tissue
- Activin signaling via ActRII receptors directly promotes lipid storage, acting as a key driver of visceral fat accumulation and obesity.
- Bimagrumab is a potent first-in-class monoclonal antibody that blocks ActRII signaling in adipose cells, mobilizing and metabolizing fat.
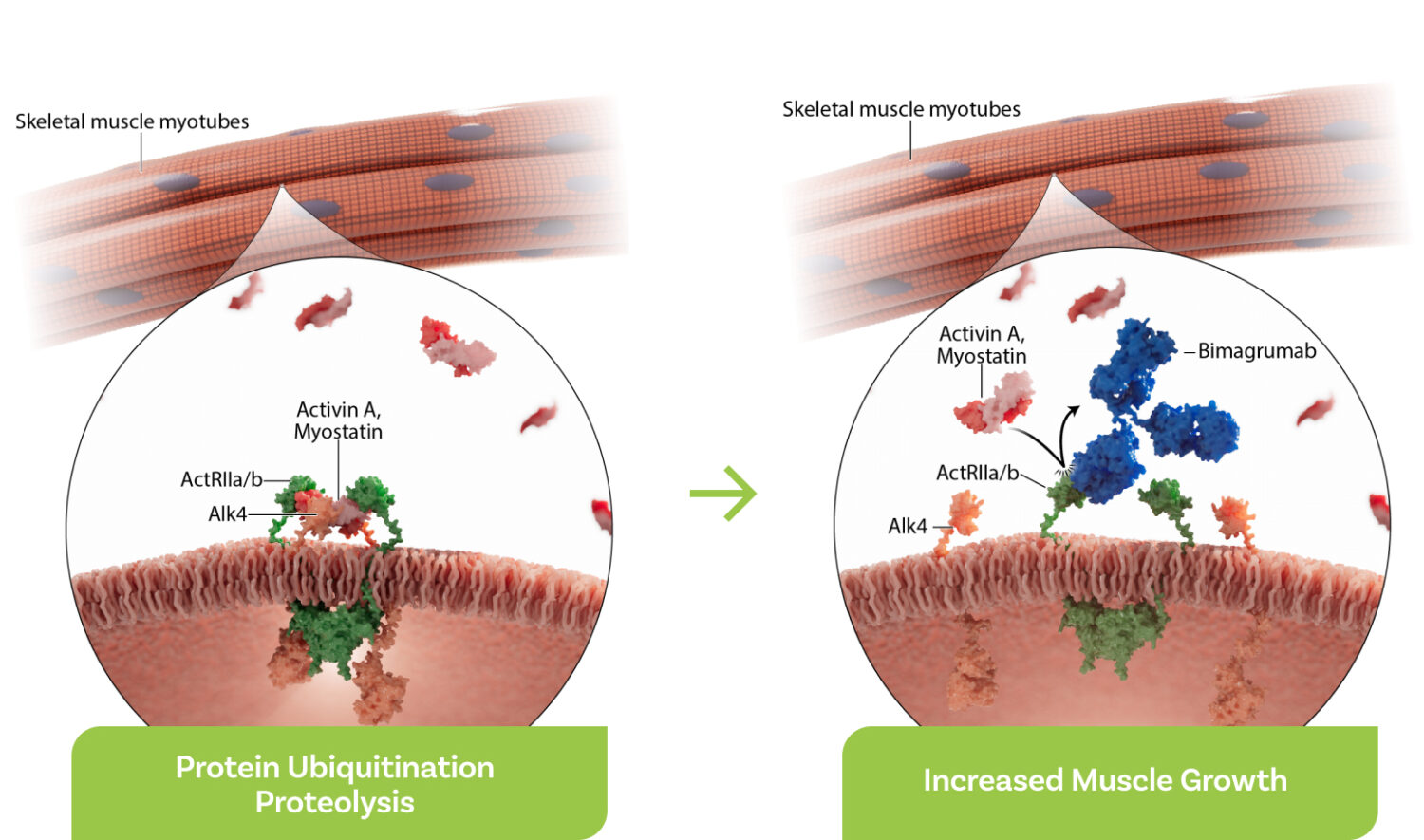
ActRII biology in muscle tissue
- Signaling via ActRII receptors inhibits muscle growth and promotes atrophy.
- Blocking activin signaling in skeletal muscles inhibits this atrophy and can promote increases in muscle mass, helping patients with obesity improve body composition and metabolism while losing fat.
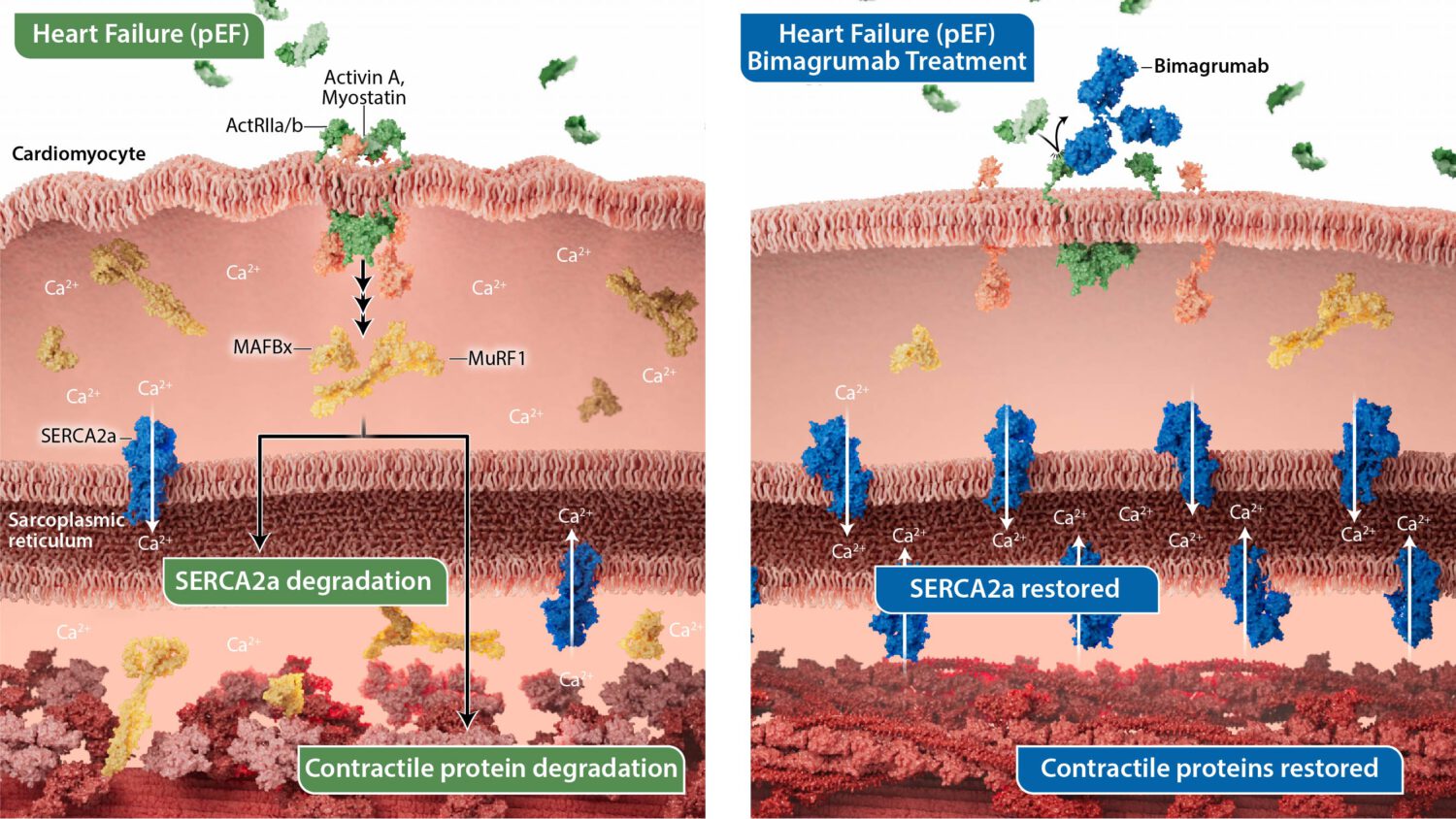
Mechanism of action in heart failure
In cardiac muscle, activin signaling has a direct mechanism in promoting contractile protein degradation. Activin signaling is elevated in patients with heart failure and is correlated with disease severity.
ActRII biology in cardiac tissue
- Activin signaling in cardiac tissue promotes degradation of cardiomyocyte proteins leading to degradation of contractile tissue.
- Bimagrumab blocks activin signaling, restoring calcium flux to the sarcoplasmic reticulum and preventing degradation of contractile proteins.